Answer:
w = -28.8 kJ/kg
q = 723.13 kJ/kg
Step-by-step explanation:
Given :
Initial properties of piston cylinder assemblies
Temperature,
 = -20°C
= -20°C
Quality, x = 70%
= 0.7
Final properties of piston cylinder assemblies
Temperature,
 = 180°C
= 180°C
Pressure,
 = 6 bar
= 6 bar
From saturated ammonia tables at
 = -20°C we get
= -20°C we get
 =
=
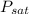 = 1.9019 bar
= 1.9019 bar
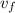 = 0.001504
= 0.001504
 / kg
/ kg
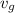 = 0.62334
= 0.62334
 / kg
/ kg
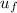 = 88.76 kJ/kg
= 88.76 kJ/kg
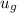 = 1299.5 kJ/kg
= 1299.5 kJ/kg
Therefore, for initial state 1 we can find
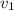 =
=
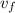 +x (
+x (
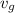 -
-
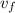
= 0.001504+0.7(0.62334-0.001504)
= 0.43678
 / kg
/ kg
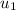 =
=
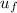 +x (
+x (
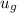 -
-
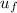
= 88.76+0.7(1299.5-88.76)
=936.27 kJ/kg
Now, from super heated ammonia at 180°C, we get,
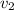 = 0.3639
= 0.3639
 / kg
/ kg
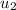 = 1688.22 kJ/kg
= 1688.22 kJ/kg
Therefore, work done, W = area under the curve
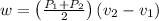
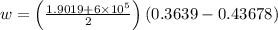
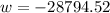 J/kg
J/kg
= -28.8 kJ/kg
Now for heat transfer

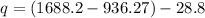
= 723.13 kJ/kg