Answer:
The temperature at which observed heat is 400 K
Step-by-step explanation:
Given data:
rejection reservoir temperature at exit
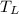 is 300 k
is 300 k
the efficiency of a engine is η = 25%
we know that efficiency of Carnot is given as
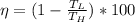
Putting all value to obtained temperature at which observed heat
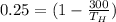
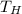 = 400 K
= 400 K