Answer:
a).Final temperature,
 = 180°C
= 180°C
b).Initial Volume,
 = 0.713412
= 0.713412

Final Volume,
 = 0.33012
= 0.33012

c). Initial enthalpy,
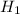 =9129.68 kJ
=9129.68 kJ
Final enthalpy,
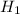 =6234.76 kJ
=6234.76 kJ
Step-by-step explanation:
Given :
Total mass, m= 2.8 kg
Initial temperature,
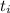 = 400°C
= 400°C
Initial pressure,
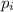 = 1.2 MPa
= 1.2 MPa
Therefore from steam table at 400°C, we can find--
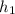 = 3260.6 kJ/kg
= 3260.6 kJ/kg
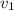 = 0.25479
= 0.25479
 / kg
/ kg
Now it is mentioned that 28% of the mass is condensed into liquid.
So, mass of liquid,
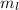 = 0.28 of m
= 0.28 of m
= 0.28 m
mass of vapour,
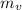 = 0.72 m
= 0.72 m
∴ Dryness fraction, x =
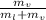
=
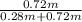
= 0.72
a). The final temperature can be evaluated from the steam table at 1.2 MPa,
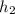 = 2226.7 kJ/kg
= 2226.7 kJ/kg
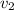 = 0.1179
= 0.1179
 / kg
/ kg
Final temperature,
 = 180°C
= 180°C
b). We know
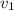 = 0.25479
= 0.25479
 / kg
/ kg
∴ Initial Volume,
 =
=
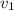 x m
x m
 = 0.25479 x 2.8
= 0.25479 x 2.8
 = 0.713412
= 0.713412

We know,
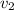 = 0.1179
= 0.1179
 / kg
/ kg
∴ Final Volume,
 =
=
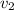 x m
x m
 = 0.1179 x 2.8
= 0.1179 x 2.8
 = 0.33012
= 0.33012

c). We know,
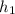 = 3260.6 kJ/kg
= 3260.6 kJ/kg
∴ Initial enthalpy,
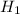 =
=
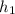 x m
x m
= 3260.6 x 2.8
= 9129.68 kJ
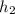 = 2226.7 kJ/kg
= 2226.7 kJ/kg
∴ Final enthalpy,
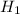 =
=
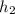 x m
x m
= 2226.7 x 2.8
= 6234.76 kJ