Answer:
m= 2.97 Kg
V₂= 3.28 m³
Step-by-step explanation:
V₁=2.5 m³ P₁= 175 kPa T₁=240 ⁰C = 240+273= 513 K
R=0.287 (characteristic gas constant)
according to ideal gas equation
P₁V₁=mRT₁
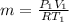
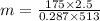
m= 2.97 Kg
T₂=400 °C= 400+273 = 673 K P₁=P₂= 175 kPa
P₂V₂=mRT₂
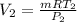
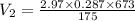
V₂= 3.28 m³