Answer:
143.5 KJ
Step-by-step explanation:
We have given mass=5 kg
vlaue of R Which is a constant =287 J/kgk
P=50 KPa
T₁=0°C=273K
T₂=100°C=100+273=373K
we know the standard equation of ideal gas=PV=mRT
Here we have to calculate volume using above equation
v₁=\frac{287\times 5\times 273}{50\times 1000}
v₁=7.833

for constant pressure
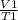 =
=
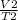
Using this formula
v₂=
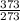 ×7.8351
×7.8351
=10.705

work done = Pdv
=50×
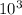 ×(10.7051-7.8351)
×(10.7051-7.8351)
=143.5 KJ