Answer:
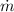 = 3.8 kg/s
= 3.8 kg/s
Step-by-step explanation:
From steam table, we can find :
The enthalpy of the super heated steam at 800 kPa or 8 bar is
h₁ = 2838.6 kJ/kg
or H₁ = 2838.6 x 30 85158 kJ
Enthalpy of the saturated water at 8 bar is
h₂ =
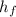 = 33.6 kJ/kg
= 33.6 kJ/kg
Now let the flow of saturated water is
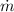
then h₂ = H₂ = 33.6
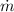 kJ
kJ
Enthalpy of dry saturated steam at 8 bar is
h₃ =
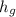 = 2516.2 kJ/kg
= 2516.2 kJ/kg
h₃ = H₃ =2516.2 x ( 30+
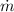 ) kJ
) kJ
Now we know from energy balance equation,
H₃ = H₁ + H₂
2516.2 x ( 30+
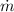 ) = 85158 + 33.6
) = 85158 + 33.6
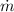
75486 + 2516.2
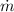 = 85158 + 33.6
= 85158 + 33.6
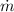
2516.2
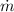 + 33.6
+ 33.6
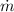 = 85158-75486
= 85158-75486
2549.8
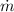 = 9672
= 9672
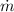 = 3.7932
= 3.7932
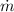 = 3.8 kg/s
= 3.8 kg/s