Answer:
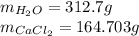
Step-by-step explanation:
Hello,
In this case, we take into account the by mass percentage as:
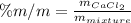
In such a way, we compute the mass of calcium chloride which is contained into 477.4g of 34.5% mixture as:
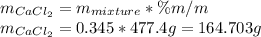
Finally, the mass of water is computed via the total mass minus the mass of calcium chloride:
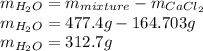
Best regards.