Answer:
14.544 g of oxygen is needed to produce 120 grams of carbon dioxide.
Step-by-step explanation:
Animals take in oxygen and breathe out carbon dioxide during cellular respiration. The reaction for the metabolism of the food in the animal body is:
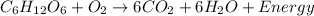
As can be seen from the reaction stoichiometry that:
6 moles of carbon dioxide gas can be produced from 1 mole of oxygen gas in the process of metabolism of glucose.
Also,
Given :
Mass of carbon dioxide gas = 120 g
Molar mass of carbon dioxide gas = 44 g/mol
The formula for the calculation of moles is shown below:

Thus, moles of carbon dioxide are:
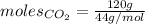
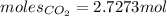
As mentioned:
6 moles of carbon dioxide gas can be produced from 1 mole of oxygen gas in the process of metabolism of glucose.
1 mole of carbon dioxide gas can be produced from 1/6 mole of oxygen gas in the process of metabolism of glucose.
2.7273 mole of carbon dioxide gas can be produced from
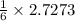 moles of oxygen gas in the process of metabolism of glucose.
moles of oxygen gas in the process of metabolism of glucose.
Thus, moles of oxygen gas needed = 0.4545 moles
Molar mass of oxygen gas = 32 g/mol
The mass of oxygen gas can be find out by using mole formula as:

Thus,
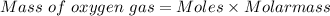
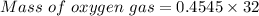
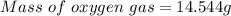
14.544 g of oxygen is needed to produce 120 grams of carbon dioxide.