Answer:
Total pressure =1.01*10^5 Pa
Step-by-step explanation:
given data:
atmospheric pressure = 1.013 *10^5 Pa\
height of water column = 60 cm
Total pressure will be sum of atmospheric pressure and pressure due to water column
P = Atmospheric pressure + pressure due to water column
pressure due to water =
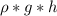
\rho of water = 1420 kg/m^{3}
height of water column = 60 cm =0.60 m
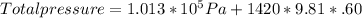
Total pressure = 109649.6 Pa = 1.10*10^5 Pa
Total pressure =1.01*10^5 Pa