Answer:
K=0.0022
Step-by-step explanation:
ΔG° = 1.22×10⁵ J/mol
Temperature (T) = 2400 K
ΔG° = - RT ln K
where R is gas constant whose value is 8.314 J/Kmol
K is equilibrium constant
ΔG° standard free energy
ln K =
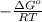
K =
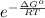
K =
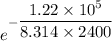
K= 0.0022
hence equilibrium constant value is K=0.0022