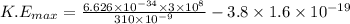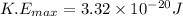Answer:
3.32×10⁻²⁰ J
Step-by-step explanation:
Given :
Wavelength of the light λ = 310 nm
work function, W₀ = 3.8 eV
The maximum kinetic energy (
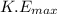 ) is given as:
) is given as:
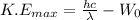
Where,
h = Planck's constant (6.626 × 10⁻³⁴ Js)
c = speed of light (3 × 10⁸)
also, 1 eV = 1.6 × 10⁻¹⁹ J
substituting the values in the above equation we get