Answer:
The the enthalpy of the reaction is 241.36 KJ.
Step-by-step explanation:
Given that,
Weight of sodium = 0.511 g
We need to calculate the number of moles
Using formula of moles
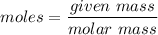
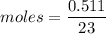
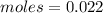
We need to calculate the energy in 1 mole
In 0.022 moles of sodium metal = 5310 J
In 1 moles of sodium metal =
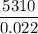
The Energy in 1 moles of sodium is 241363.63 J.
Hence, The the enthalpy of the reaction is 241.36 KJ.