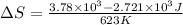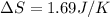Answer:
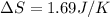
Step-by-step explanation:
We know,
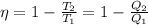 ..............(1)
..............(1)
where,
η = Efficiency of the engine
T₁ = Initial Temperature
T₂ = Final Temperature
Q₁ = Heat available initially
Q₂ = Heat after reaching the temperature T₂
Given:
η =0.280
T₁ = 3.50×10² °C = 350°C = 350+273 = 623K
Q₁ = 3.78 × 10³ J
Substituting the values in the equation (1) we get
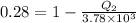
or
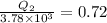
or
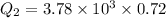
⇒
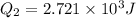
Now,
The entropy change (
 ) is given as:
) is given as:
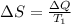
or
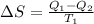
substituting the values in the above equation we get