Answer:
 =
=
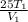
where
 is initial volume in liters
is initial volume in liters
 is initial temperature in kelvins
is initial temperature in kelvins
Step-by-step explanation:
Let the initial volume be
 and the initial temperature be
and the initial temperature be

Now by ideal gas law
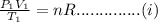
Similarly let
 be final volume
be final volume
 be the final volume
be the final volume
thus by ideal gas law we again have
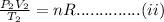
Equating i and ii we get
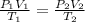
For system at constant pressure the above expression reduces to
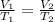
Solving for
 we get
we get
 =
=
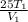
where
 is initial volume in liters
is initial volume in liters
 is initial temperature in kelvins
is initial temperature in kelvins