Answer:
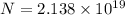 Rubidium atoms
Rubidium atoms
Step-by-step explanation:
Given:
The half life of Rubidium-87 =
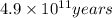
Decay rate, A = 3500 Disintegration per hr
Now, converting the half life in hrs
Half life in hrs will be = 4.9×10¹¹ yrs × 360 days/ 1yrs × 24 hrs / 1 days
Half life in hrs = 4.23×10¹⁵ hrs
Now, We know that
Activity (A) = λ N
where, N = number of atoms.
also,
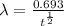
where,
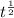 = half life
= half life
therefore,
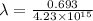
= 1.64×10⁻¹⁶ hr⁻¹
now,
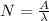
substituting the values in the equation we have
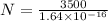
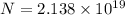 Rubidium atoms
Rubidium atoms