Answer : The order of reaction is 2 (second order) and the rate law is,
![R=k[AB]^2](https://img.qammunity.org/2020/formulas/physics/college/qov5m7e2s1xc35v2oh9euqk8au2fu0tiwm.png)
Explanation :
As the expression of rate is,
![R=k[AB]^n](https://img.qammunity.org/2020/formulas/physics/college/eq15zu1zd9qiryoby8qmnndqigcng9faxm.png)
where,
R = rate
k = rate constant
[AB] = concentration of AB
n = order of the reaction
First we have to calculate the rate for 0.200 mol/L 'AB' concentration with initial rate 0.00320 mol/L.s
![R_1=k[AB_1]^n](https://img.qammunity.org/2020/formulas/physics/college/pxsa431z6fru21we0gmvmqgmrultfvw2ja.png)
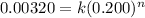 .............(1)
.............(1)
Now we have to calculate the rate for 0.400 mol/L 'AB' concentration with initial rate 0.0128 mol/L.s
![R_2=k[AB_2]^n](https://img.qammunity.org/2020/formulas/physics/college/k65m7ooc2od0jibyp51528izco7zf1k9qo.png)
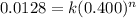 .............(2)
.............(2)
Now dividing the equation 1 by equation 2, we get the order of reaction.
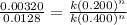
By solving the term, we get the value of 'n'.
n = 2
Thus, the order of reaction = n = 2
The rate law will be,
![R=k[AB]^2](https://img.qammunity.org/2020/formulas/physics/college/qov5m7e2s1xc35v2oh9euqk8au2fu0tiwm.png)
So we conclude that,
For n = 0, the rate law will be zero order.
For n = 1, the rate law will be first order.
For n = 2, the rate law will be second order.
and so on.....
Hence, the order of reaction is 2 (second order) and the rate law is,
![R=k[AB]^2](https://img.qammunity.org/2020/formulas/physics/college/qov5m7e2s1xc35v2oh9euqk8au2fu0tiwm.png)