Answer: The amount of silver produced in the given reaction is 459.63 g.
Step-by-step explanation:
According to mole concept:
1 mole of an atom contains
 number of atoms.
number of atoms.
For the given chemical equation:
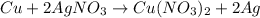
By Stoichiometry of the reaction:
1 mole of copper produces 2 moles of silver.
This means that,
 number of atoms of copper produces
number of atoms of copper produces
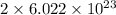 number of atoms of silver.
number of atoms of silver.
So,
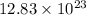 number of atoms of copper will produce =
number of atoms of copper will produce =
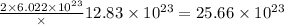 number of atoms of silver.
number of atoms of silver.
We know that:
Mass of 1 mole of silver = 107.87 g
Using mole concept:
If,
 number of atoms occupies 107.87 grams of silver atom.
number of atoms occupies 107.87 grams of silver atom.
So,
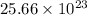 number of atoms will occupy =
number of atoms will occupy =
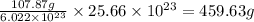
Hence, the amount of silver produced in the given reaction is 459.63 g.