Answer:
15.8 year
Step-by-step explanation:
This this a first order decay reaction first order decay reaction is that reaction in which the rate of reaction is linearly depend on the concentration of reactants. for the first order decay the equation is given as
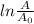 = -Kt
= -Kt
where
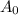 =initial activity and A is the activity at time t K is a rate constant
=initial activity and A is the activity at time t K is a rate constant
using above equation
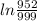 = -K×1.10
= -K×1.10
K=0.043809 /year
Half life=
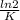
=
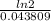
=15.8 year