Answer:
Product side
Step-by-step explanation:
When water vapor reacts reversibly with solid carbon to yield a mixture of hydrogen gas and carbon monoxide and we continually add more water vapor to the reaction the equilibrium of the reaction shifts to the product side.
Because gaseous water is reactant that appears in the reaction quotient expression.
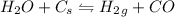
When we add more water vapor to the reaction the product formation is increased. The reaction goes in forward direction affecting the equilibrium.