Answer:
rate of change in temperature of copper is more than the rate of change in temperature of aluminium.
so here copper will reach to our body temperature first
Step-by-step explanation:
As we know that rate of energy absorb by the two sphere is same
so here we will have
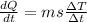
now for copper sphere we will have
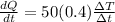
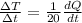
now for Aluminium sphere we will have
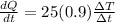
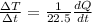
So rate of change in temperature of copper is more than the rate of change in temperature of aluminium.
so here copper will reach to our body temperature first