Answer : The value of entropy change will be, 29.8455 J/K
Explanation : Given,
Moles of
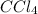 = 0.35 mole
= 0.35 mole
Boiling temperature =
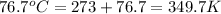
conversion used :
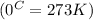
Molar enthalpy of vaporization of
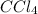 = 29.82 kJ/mole
= 29.82 kJ/mole
First we have to calculate the heat change.
Formula used :
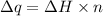
where,
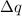 = heat change
= heat change
 = molar enthalpy of vaporization
= molar enthalpy of vaporization
n = number of moles
Now put all the given values in the above formula, we get:
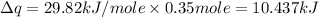
Now we have to calculate the entropy change.
Using third law of thermodynamic :
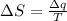
where,
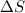 = entropy change
= entropy change
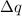 = heat change
= heat change
T = boiling temperature
Now put all the given values in this formula, we get:
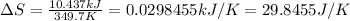
Therefore, the value of entropy change will be, 29.8455 J/K