Answer:
1.27 x 10⁵ L
Step-by-step explanation:
V₁ = initial volume = 14100 L
V₂ = final volume = ?
P₁ = initial pressure = 745 torr
P₂ = final pressure = 63.1 torr
T₁ = initial temperature = 21 °C = 21 + 273 = 294 K
T₂ = final temperature = - 48 °C = - 48 + 273 = 225 K
Using the equation
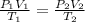
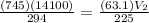
 = 1.27 x 10⁵ L
= 1.27 x 10⁵ L