Answer:
C. 26.4 kJ/mol
Step-by-step explanation:
The Chen's rule for the calculation of heat of vaporization is shown below:
![\Delta H_v=RT_b\left [ (3.974\left ( (T_b)/(T_c) \right )-3.958+1.555lnP_c)/(1.07-\left ( (T_b)/(T_c) \right )) \right ]](https://img.qammunity.org/2020/formulas/chemistry/college/jv54tna65tste3z41aydnsm0k4culn762w.png)
Where,
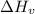 is the Heat of vaoprization (J/mol)
is the Heat of vaoprization (J/mol)
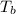 is the normal boiling point of the gas (K)
is the normal boiling point of the gas (K)
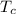 is the Critical temperature of the gas (K)
is the Critical temperature of the gas (K)
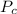 is the Critical pressure of the gas (bar)
is the Critical pressure of the gas (bar)
R is the gas constant (8.314 J/Kmol)
For diethyl ether:
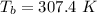
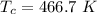
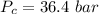
Applying the above equation to find heat of vaporization as:
![\Delta H_v=8.314*307.4 \left [ (3.974\left ( (307.4)/(466.7) \right )-3.958+1.555ln36.4)/(1.07-\left ( (307.4)/(466.7) \right )) \right ]](https://img.qammunity.org/2020/formulas/chemistry/college/cdx8nxmzank9f272gbjg2pxvspmb2almdv.png)
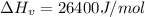
The conversion of J into kJ is shown below:
1 J = 10⁻³ kJ
Thus,
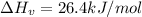
Option C is correct