Answer:
Mass of nitrogen gas dissolved= 1.1732 grams
Step-by-step explanation:
According to Dalton's Law of partial pressure:
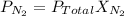
Where,
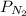 is the partial pressure of nitrogen
is the partial pressure of nitrogen
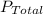 is the Total pressure
is the Total pressure
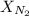 is the mole fraction of nitrogen
is the mole fraction of nitrogen
Given :
Total pressure = 1.0 atm
Mole fraction of nitrogen = 0.78
Partial pressure of nitrogen:
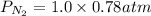
Partial pressure of nitrogen = 0.78 atm
According to Henry's law:
Solubility = Henry's constant (k)×Partial pressure
k = 6.26×10⁻⁴ mol/L-atm
Thus,
Solubility of nitrogen = 6.26×10⁻⁴ mol/L-atm×0.78 atm = 4.8828×10⁻⁴ mol/L
Given: Volume = 86.0 L
So, Moles of nitrogen gas dissolved:
Moles = Solubility (Concentration dissolved)×Volume
Moles = 4.8828×10⁻⁴ mol/L×86.0 L = 0.0419 moles
Also,
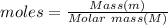
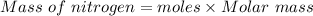
Molar mass of nitrogen gas = 28 g/mol
So,
Mass of nitrogen gas dissolved = 0.0419 moles×28 g/mol = 1.1732 grams