Answer: 2.6 ml of chlorpromazine are administered.
Step-by-step explanation:
Given stock solution of chlorpromazine, 3.2 %(m/v) i.e 3.2 grams of chlorpromazine are dissolved in 100 ml of solution
We need to find the volume of solution that can contain 0.083 grams of chlorpromazine.
By applying unitary method, we get:
3.2 grams of chlorpromazine is present in 100 mL of solution.
So, 0.083 grams of chlorpromazine will be present in =
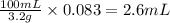 of solution.
of solution.
Hence, the given amount of chlorpromazine will be present in 2.6 mL of solution and thus 2.6 ml are administered.