Answer:
Rhodium has FCC structure.
Step-by-step explanation:
Formula used :
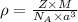
where,
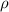 = density
= density
Z = number of atom in unit cell
M = atomic mass
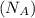 = Avogadro's number
= Avogadro's number
a = edge length of unit cell
1) If it FCC cubic lattice
Number of atom in unit cell of FCC (Z) = 4
Atomic radius of Rh= 0.1345 nm =
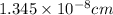
Edge length = a
For FCC, a = 2.828 × r :
a =
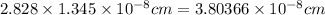
Density of Rh=
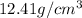
Atomic mass of Rh(M) = 102.91 g/mol
On substituting all the given values , we will get the value of 'a'.
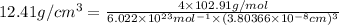
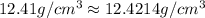
2) If it BCC cubic lattice
Number of atom in unit cell of BCC (Z) = 2
Atomic radius of Rh= 0.1345 nm =
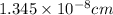
Edge length = a
For BCC, a = 2.309 × r :
a =
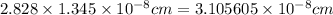
Density of Rh=
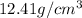
Atomic mass of Rh(M) = 102.91 g/mol
On substituting all the given values , we will get the value of 'a'.
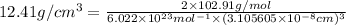
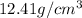 ≠
≠
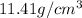
Rhodium has FCC structure.