Answer:
pH = 11.37
Step-by-step explanation:
Sodium cyanide will dissociate into sodium ion and cyanide ion. This cyanide ion will get hydrolyzed.The ICE table for hydrolysis of cyanide ion is:

Initial 0.265 0 0
Change -x +x +x
Equilibrium 0.265-x x x
![K=([HCN][OH^(-))/([CN^(-)])](https://img.qammunity.org/2020/formulas/chemistry/college/b9qanec69cr4ba9uxuhy353oa2yl8nylaz.png)
This K is Kb of HCN
Kb = Kw / Ka
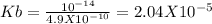
Putting values

x can be ignored in denominator as Kb is very low
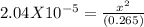
x= 2.33X10⁻³ M = [OH⁻]
pOH = -log[OH⁻]
pOH = -log(2.33X10⁻³ )
pOH = 2.63
pH = 14- pOH = 14-2.63 = 11.37