Answer : The temperature of this gas will be, 206.9 K
Explanation :
The expression for the kinetic energy per molecule of monoatomic gas (argon) is:
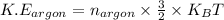 ...........(1)
...........(1)
The expression for the kinetic energy per molecule of diatomic gas (nitrogen gas) is:
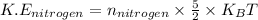 .............(2)
.............(2)
The total kinetic energy of the molecule will be,
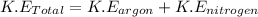
Now put all the expression in this, we get:
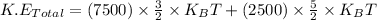
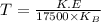
Now put all the given values in this expression, we get:
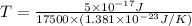
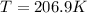
Therefore, the temperature of this gas will be, 206.9 K