Answer:In adiabatic case
Step-by-step explanation:
Let us suppose Initial condition
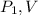
be the initial pressure and volume of gas
and
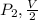
be the final pressure
 In Isothermal case
In Isothermal case
Temprature is costant therefore
PV=constant
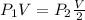

for adiabatic case
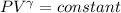
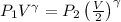
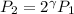
and we know
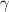 is greater than 1
is greater than 1
therefore In adiabatic case pressure will be high.