Answer : The correct option is, (b) unsaturated
Explanation :
Ionic product : It is defined as the product of the concentrations of the ions present in solution raised to the same power by its stoichiometric coefficient in a solution of a salt. This takes place at any concentration. The ionic product is represented as, Q.
Solubility product constant : It is defined as the product of the concentration of the ions present in a solution raised to the power by its stoichiometric coefficient in a solution of a salt. This takes place at equilibrium only. The solubility product constant is represented as,
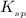 .
.
Now we have to calculate the ionic product of given solution.
The balanced chemical reaction is,

The expression of ionic product will be,
![Q=[Pb^(2+)][Cl^-]^2](https://img.qammunity.org/2020/formulas/chemistry/college/a5i72wcolh52zhy6ntusf8ifmu8w8wxric.png)
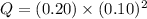

The ionic product of solution is, 0.002
There are three cases for the solubility :
When
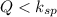 this means that the solution is unsaturated solution and more solid will be dissolve.
this means that the solution is unsaturated solution and more solid will be dissolve.
When
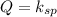 this means that the solution is saturated solution.
this means that the solution is saturated solution.
When
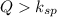 this means that the solution is supersaturated solution and solid will be precipitate.
this means that the solution is supersaturated solution and solid will be precipitate.
From this we conclude that the value of
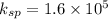 is greater than the ionic product that means the solution is unsaturated solution and more solid will be dissolve.
is greater than the ionic product that means the solution is unsaturated solution and more solid will be dissolve.
Hence, the correct option is, (b) unsaturated