Answer:
The average kinetic energy of a gas depends only on its temperature.
Step-by-step explanation:
The average kinetic energy of particles in a gas can be found using the equation
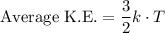 ,
,
where
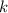 is the Stefan-Boltzmann constant, and
is the Stefan-Boltzmann constant, and
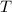 is the absolute temperature of this gas (the one in degree Kelvins.)
is the absolute temperature of this gas (the one in degree Kelvins.)
As seen in this equation, the average kinetic energy of particles in a gas depends only on the temperature of the gas. Also, since the question is asking for the average not the total kinetic energy, the number of particles in this gas doesn't matter, either.