Answer:
1184 kJ/kg
Step-by-step explanation:
Given:
water pressure P= 28 bar
internal energy U= 988 kJ/kg
specific volume of water v= 0.121×10^-2 m^3/kg
Now from steam table at 28 bar pressure we can write
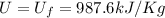
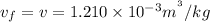
therefore at saturated liquid we have specific enthalpy at 55 bar pressure.
that the specific enthalpy h = h at 50 bar +(55-50)/(60-50)*( h at 50 bar - h at 60 bar)
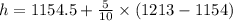
h= 1184 kJ/kg