Hello!
The answer is:
There are 0.209 moles of Cu in 13.g of Cu
Why?
To calculate how many moles does a sample of any element has, we need to use its atomic mass
We are working with Copper (Cu), so we need to find its atomic mass to calculate how many moles does 13.3 g of Cu contains.
So, calculating we have:
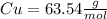
We have that there is 1 mol per 63.54 grams of Cu.
Now, converting we have:
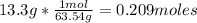
We have that there are 0.209 moles of Cu in 13.g of Cu
Have a nice day!