Answer: Option (b) is the correct answer.
Step-by-step explanation:
As it is known that more is the kinetic energy present between the molecules of a gas, more will be the number of collisions take place.
Also, relation between kinetic energy and temperature is as follows.
K.E =
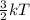
This means that more is the temperature of gas more will be the kinetic energy present withing its molecules or vice-versa.
As methane is at 340 K.
Carbon dioxide is at 80
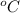 = (80 +273) K = 353 K
= (80 +273) K = 353 K
Argon is at 265 K and helium is at 20
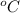 = (20 + 273)K = 293 K
= (20 + 273)K = 293 K
Therefore, temperature of carbon dioxide gas is the maximum.
Thus, we can conclude that the carbon dioxide gas at 80
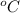 will have the most collisions between its particles.
will have the most collisions between its particles.