Answer : The correct option is, (B) 9150 joules
Explanation : Given,
Initial temperature =
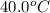
Final temperature =
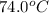
Mass of water = 64.3 g
Specific heat of water =
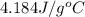
Formula used :
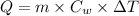
or,
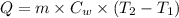
where,
Q = heat absorbed = ?
m = mass of water
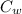 = specific heat of water
= specific heat of water
 = initial temperature
= initial temperature
 = final temperature
= final temperature
Now put all the given value in the above formula, we get:
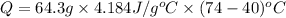
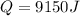
Therefore, the heat absorbed will be, 9150 J