Answer:
This ion is in an octahedral geometry.
See the diagram attached for the Lewis Dot Structure of the iodide hexafluoride cation
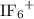 . (Created with Google Drawings.)
. (Created with Google Drawings.)
Note that in this diagram,
- A pair of double dots on an atom represent a lone pair of electrons.
- A single dash represents a single chemical bond.
- The square bracket and the superscript indicates that this structure is charged.
Step-by-step explanation:
The iodine in
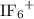 forms an expanded octet. There are twelve valence electrons in total around this atom.
forms an expanded octet. There are twelve valence electrons in total around this atom.
- Each of the six fluorine atom needs 8 - 7 = 1 electron to achieve an octet of eight electrons.
- The iodine atom needs 12 - 7 = 5 electrons to achieve an expanded octet of twelve electrons.
- The ion carries a positive charge of +1. Atoms in this ion lacks one extra electron.
Overall, there needs to be
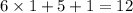 more electrons for seven atoms to achieve an octet. They will form half that number of chemical bonds. That's
more electrons for seven atoms to achieve an octet. They will form half that number of chemical bonds. That's
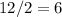 bonds.
bonds.
Now consider: what will be the geometry of this ion? There are six chemical bonds but no lone pair around the central iodine atom. The six
 bonds repel each other equally. They will stay as far apart from each other as possible. As a result, the shape of the ion will be octahedral. Each of the fluorine atoms occupies a vertex.
bonds repel each other equally. They will stay as far apart from each other as possible. As a result, the shape of the ion will be octahedral. Each of the fluorine atoms occupies a vertex.