Answer:
42.65g
Step-by-step explanation:
Given parameters:
Mass of K = 4g
Unknown: Mass of KCl
Solution:
Complete equation of the reaction:
2K + Cl₂ → 2KCl
To solve this problem, we know that the reactant in short supply is potassium K and this dictates the amount of products that would be formed. The chlorine gas is in excess and we can't use it to determine the amount of product that would form.
Now, we work from the known to the unknown. Since we know the mass of K given in the reaction, we can simply find the molar relationship between the reacting potassium and the product. We simply convert the mass to mole and compare to the product. From there we can find the mass of KCl that would be produced.
Calculating number of moles of K
Number of moles =
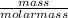
Number of moles of K =
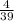 = 0.103mol
= 0.103mol
From the given reaction equation:
2 moles of K will produce 2 moles of KCl
Therefore 0.103mol of K will produce 0.103mol of KCl
To find the mass of KCl produced,
Mass of KCl = number of moles of KCl x molar mass
Molar mass of KCl = 39 + 35.5 = 74.5gmol⁻¹
Mass of KCl = 0.103 x 74.5 = 42.65g