Answer:
a. forming bonds
Step-by-step explanation:
The energy required to break a bond is endothermic that is energy is absorbed to break a bond.
The energy is released in the formation of a bond that is energy is released when a bond is formed.
The formula to find the ∆H of the reaction is
∆H (reaction) = ∆H (bonds Broken) - ∆H (bonds formed)
For example
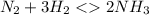
 contains one N≡N triple bond (Bond breaking 946 KJ per mol)
contains one N≡N triple bond (Bond breaking 946 KJ per mol)
 contains a single H-H bond (bond breaking 436 KJ per mol)
contains a single H-H bond (bond breaking 436 KJ per mol)
 contains 3, N - H single bonds(389 KJ per mol)
contains 3, N - H single bonds(389 KJ per mol)
So ∆H (bonds broken) = 946 + (3 × 436) = 2254KJ
∆H (Bonds formed ) = (2 × 3 × 389) = 2334KJ
So
∆H (reaction) = 2254 KJ - 2334 KJ
= - 80KJ and the reaction is Exothermic
In this example we see energy required to break the bond is lesser than energy released in forming the bond.