Answer:
1119.1 K
Step-by-step explanation:
From Clausius-Clapeyron equation:
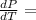 Δ
Δ
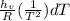
The equation may be integrated considering the enthalpy of vaporization constant, and its result is:
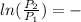 Δ
Δ
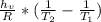
Isolating the temperature

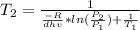
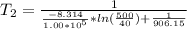
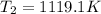
Note: Remember to change the units of the enthalpy vaporization to J/mol; and the temperatures must be in Kelvin units.
There is a format mistake with the enthalpy of vaporization, each 'Δ' correspond to that.