Answer: A.
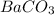
Step-by-step explanation:
A double displacement reaction is one in which exchange of ions take place.
The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
The balanced chemical equation will be given as:
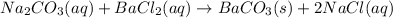
Thus the insoluble precipitate formed is
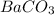 .
.