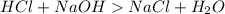Answer:
B. Decomposition
Step-by-step explanation:
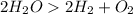
Decomposition is the type of reaction in which a compound is broken into smaller compounds or broken into its elements
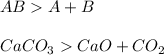
Synthesis is just opposite to decomposition reaction, Elements combine to form a compound
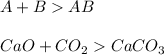
Single replacement reactions in which replacement takes place at a single place
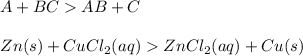
Double replacement reactions in which replacement occurs two places
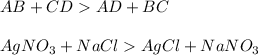
Neutralization reaction is the reaction between an acid and a base to form salt and water