Answer:
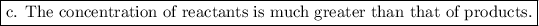
Step-by-step explanation:
NO₂ + NO₃ ⇌ N₂O₅; K = 2.1 × 10⁻²⁰
We often write K as
![K = \frac{[\text{Products}]}{[\text{Reactants}]}](https://img.qammunity.org/2020/formulas/chemistry/college/s7tx6r7u76is7fkfis6edr0ahjaitxjsto.png)
If K is large, more of the molecules exist as products.
If K is small, more of the molecules exist as reactants.
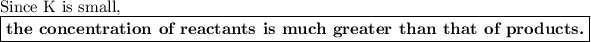
a. b., and d. are wrong. The concentration of reactants is greater.