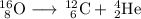Answer:
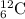
Step-by-step explanation:
A particle with two protons and two neutrons is a helium nucleus.
Your unbalanced nuclear equation is:
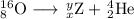
The main point to remember in balancing nuclear equations is that the sums of the superscripts and of the subscripts must be the same on each side of the equation.
Then
8 = x + 2, so x = 8 - 2 = 6
16 = y + 4, so y = 16 - 4 =12
Element 6 is carbon, so the nuclear equation becomes