Answer:
A. 52.8
Step-by-step explanation:
We make use of the dilution formula to solve this
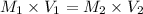
Where (
 and
and
 are the initial molarity and final molarity and
are the initial molarity and final molarity and
 and
and
 are the initial volume and final volume)
are the initial volume and final volume)
Plugging into the values in the formula
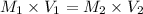
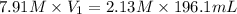
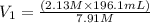
= 52.8 mL is the Answer