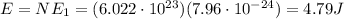Answer:
4.79 J
Step-by-step explanation:
The energy of a single photon is given by
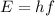
where
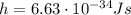 is the Planck constant
is the Planck constant
f is the frequency of the photon
Here we have
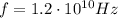
so the energy of one photon is
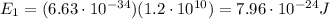
Here we have 1 mol of photons, which contains
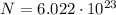 photons (Avogadro number). So, the total energy of this mole of photons is:
photons (Avogadro number). So, the total energy of this mole of photons is: