Answer:
5.4 x 10²²molecules
Step-by-step explanation:
Given parameters = 2L
Unknown; number of molecules
Condition of the water vapor is at standard Temperature and Pressure
Solution
To solve this problem, we simply find the number of moles the given volume would yield at STP using the relationship below:
Number of moles =
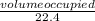
Number of moles =
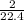 = 0.089mole
= 0.089mole
To find the number of molecules in the solved mole of the water vapor:
we use the avogadro's constant to evaluate.
1 mole of a water vapor = 6.02 x 10²³ molecules
0.089 mole of water vapor = 0.54 x 10²³molecules
Number of molecules of water is 5.4 x 10²²molecules