Answer:
3626.76dm³
Step-by-step explanation:
Given parameters:
Number of moles of Nitrogen in tank = 17moles
Temperature of the gas = 34°C
Pressure on the gas = 12000Pa
Unkown:
Volume of the tank, V =?
Converting the parameters to workable units:
We take the temperature from °C to Kelvin
K = 273 + °C = 273 + 34 = 307k
Taking the pressure in Pa to atm:
101325Pa = 1atm
12000Pa = 0.118atm
Solution:
To solve this problem, we employ the use of the ideal gas equation. The ideal gas law combines three gas laws which are the Boyle's law, Charles's law and the Avogadro's law.
It is expressed as PV = nRT
The unknown is the Volume and we make it the subject of the formula
V =
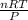
Where R is called the gas constant and it is given as 0.082atmdm³mol⁻¹K⁻¹
Therefore V =
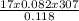 = 3626.76dm³
= 3626.76dm³