Answer:
8.38g
Step-by-step explanation:
Given parameters:
Volume of the gas = 12L or 12dm³
Temperature = 293k
Pressure = 100kPa
Unknown: mass of the ammonia gas = ?
_____ Now let us take the unit of pressure to atm:
Pressure = 100 kPa
1kPa = 0.00986923atm
100kPa = 100 x 0.00986923 = 0.987atm
Solution:
From the ideal gas law, we can find the number of moles of the ammonia gas. Using the number of moles, it would be possible to find the mass of the ammonia.
PV = nRT
n =
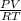
Where R is the gas constant and its value is 0.082atmdm³mol⁻¹K⁻¹
n =
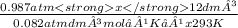
n = 0.493mol
We know that, mass = number of moles x molar mass
Molar mass of NH₃ = 14 + (3x1) = 17gmol⁻¹
mass of NH₃ = 0.493 x 17 = 8.38g