Answer:
Approximately
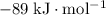 .
.
Assumption: the density of the solution is equal to the density of pure water.
Step-by-step explanation:
The enthalpy of neutralization is defined as the enthalpy change for each moles of water produced. (Clark, Physical & Theoretical Chemistry, Chemistry Libretexts.)
Each mole of
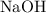 formula units will neutralize one mole of
formula units will neutralize one mole of
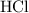 to produce one mole of water.
to produce one mole of water.
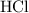 and
and
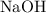 are available at equal volume and concentration. In other words, there's an equal number of both reactants. All
are available at equal volume and concentration. In other words, there's an equal number of both reactants. All
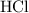 and
and
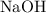 will react to form water.
will react to form water.
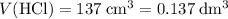 .
.
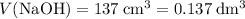 .
.
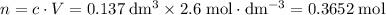 .
.
In other words, there are
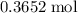 of
of
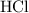 and
and
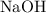 each. The two will react to produce
each. The two will react to produce
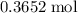 of water.
of water.
How much heat is released?
Assume that the volume of the liquid is equal to the volume of the
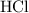 solution plus the volume of the
solution plus the volume of the
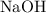 solution. That's
solution. That's
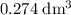 . Assume that the density of the solution is equal to that of water under room temperature.
. Assume that the density of the solution is equal to that of water under room temperature.
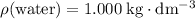 . The mass of the liquid will be
. The mass of the liquid will be
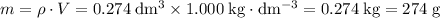 .
.
Change in temperature:
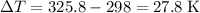 .
.
Heat that the solution absorbed:
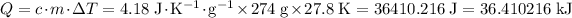 .
.
That will also be the amount of heat released from the reaction if there's no energy loss.
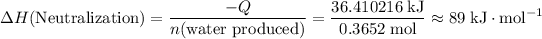 .
.