Answer:
The final temperature is 79.16°C.
Step-by-step explanation:
Given that,
Heat
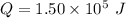
Temperature = 20.0°C
Entropy = 465 J/k
We need to calculate the average temperature
Using relation between entropy and heat
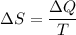
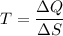
Where, T = average temperature
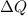 = transfer heat
= transfer heat
 = entropy
= entropy
Put the value into the formula
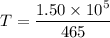
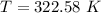
We need to calculate the final temperature
Using formula of average temperature
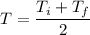
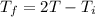 ....(I)
....(I)
Put the value in the equation (I)
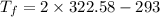
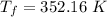
We convert the temperature K to degrees
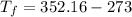
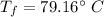
Hence, The final temperature is 79.16°C.